A review of the effects of processing methods and modern preservation strategies (Part 1)
Lycopene, one of the most frequent carotenoids in the human diet, is a well-known natural compound that provides protective effects against chronic diseases. As industrial and domestic processing and storage conditions significantly influence the retention and isomerization of lycopene, a lot of attention has been paid to the preservative effects of lycopene in the past few years.
A review has been carried out highlighting recent strategies that have been developed to preserve lycopene in processed products, particularly in tomato pulp, puree, paste and juice. The key factors influencing lycopene degradation and isomerization, such as other ingredients and the intensity of thermal treatments, are also discussed. Special attention has been paid to the crystalline structures of lycopene, which facilitate its resistance to degradation and isomerization.
Emerging non-thermal processing methods, such as ultrasound and high-pressure processing (HPP), are critically evaluated for their preservation of thermo-labile compounds.
Novel trends to improve lycopene stability by micro- and nano-encapsulation and the addition of antioxidants are also included in order to examine their potential for protecting against the effects of light, heat, metals (e.g. Fe3+) and other oxidative processes.
Finally, recommended processing and storage conditions are discussed in order to provide strategies that contribute to retaining the highest possible amount of bioactive lycopene until consumption.
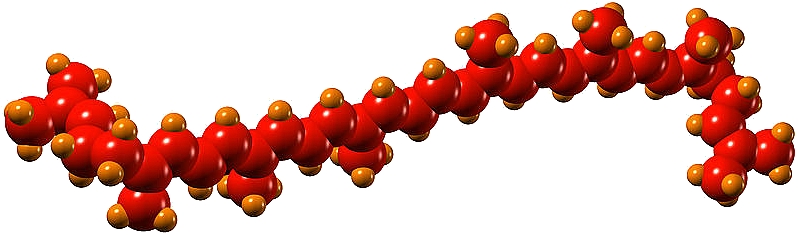
Lycopene is a polyene, which belongs to a wider group of carotenoid pigments synthesized by plants and photosynthetic microorganisms. Several mechanistic and epidemiological studies have demonstrated the pronounced beneficial role of dietary lycopene intake in decreasing the risk of chronic and non-communicable diseases, including neurodegenerative disorders, obesity, type 2 diabetes, cancer, and cardiovascular diseases. Principally, these diseases are associated with persistent low-grade inflammation induced by cellular oxidative stress. Owing to its potent antioxidant properties at the cellular level, lycopene can potentially normalize the levels of proinflammatory mediators by helping to balance the oxidative stress. Thanks to the health-promoting properties of lycopene and other antioxidants in fruit, vegetables and cooked tomato sauces, these ingredients are regarded as key components of the Mediterranean diet, known for its health benefits thanks to dishes like sofrito (a sauce mainly consisting of onion, tomatoes and peppers cooked in olive oil). With their red color, ripened tomatoes, watermelons, guavas, papayas, and grapefruits are key sources of lycopene in the diet.
Since tomatoes are the most widely processed and consumed crop among these sources, fresh tomatoes and tomato-based commercial products are the primary sources of dietary lycopene. A series of processing steps such as hot/cold breaking, evaporation, pasteurization, sterilization, and canning are commonly used in tomato processing. Under these conditions, lycopene may undergo degradation via oxidation and isomerization. In particular, thermal processing such as blanching, cooking, drying, pasteurization, sterilization and canning may reduce the total lycopene content in processed foods, resulting in a direct negative influence on the antioxidant potential and health benefits of processed products.
However, the disruption of food matrices (e.g. cellulose-thickened cell walls and membranes) during processing may enhance the release and solubilization of lycopene, which results in enhanced bioaccessibility and bioavailability. Moreover, processing conditions are known to accelerate the isomerization of all-E-forms (which have low bioaccessibility) to Z-forms of lycopene, which are highly bioaccessible and bioactive. Considering these facts, processing methods play a critical role in lycopene’s biological activity and bioavailability. Thus, in recent years, great attention has been paid to emerging processing technologies (e.g. high pressure and ultrasound processing), which may positively influence lycopene retention and accessibility in processed food products. Moreover, processing conditions that promote all-E-form to Z-form isomerization have been emerging in recent years and are worthy of further discussion.
There are several studies that have examined the effects of processing and storage conditions on lycopene. However, it is still a challenge to develop clear strategies for enhancing the processing and storage stability of lycopene. Contrasted findings have been reported. For instance, researchers Gupta and Colle reported no significant lycopene degradation and isomerization during typical tomato processing, while Capanoglu et al. and Koh et al. indicated significant degradation of lycopene and Z-lycopene isomer formation after thermal processing of tomato. Thus, up-to-date insights are needed to reveal the major causes of these discrepancies. The review (see attached document) evaluates recent findings on qualitative and quantitative changes in lycopene content (primarily due to isomerization and degradation) during the processing and storage of lycopene-rich fruit and vegetables, especially tomatoes. Subsequently, an attempt is made to clarify the controversial issues surrounding the possible causes for these contrasting observations on lycopene retention.
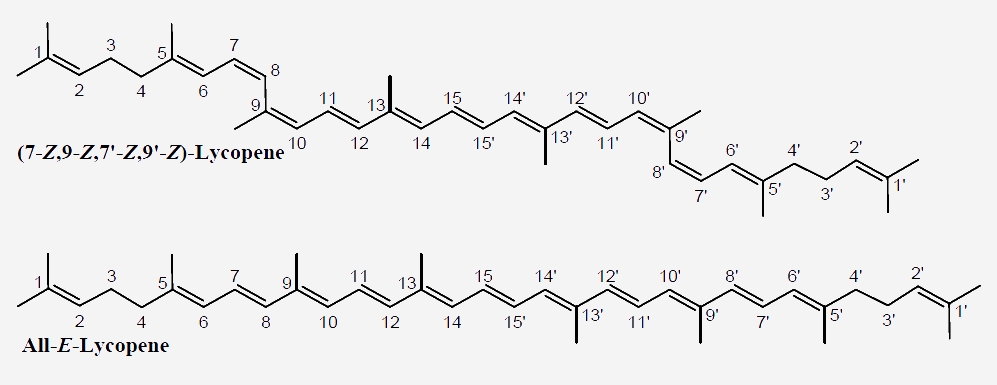
Chemical structure of various lycopene isomers identified in different foods.
Lycopene (Ψ, Ψ-carotene) is a symmetrical C40 tetraterpenoid hydrocarbon, comprised of eight C5 isoprene units chemically bound by head-to-tail coupling reactions (1-4 linkage), except for the central unit, which is bound by tail-to-tail or 4-4 linkage. The lycopene molecule bears 2 unconjugated 11 conjugated double bonds, creating an electron-rich environment responsible for the exceptional antioxidant activities. At the same time, the presence of several conjugated bonds makes the compounds highly sensitive to degradation and isomerization.
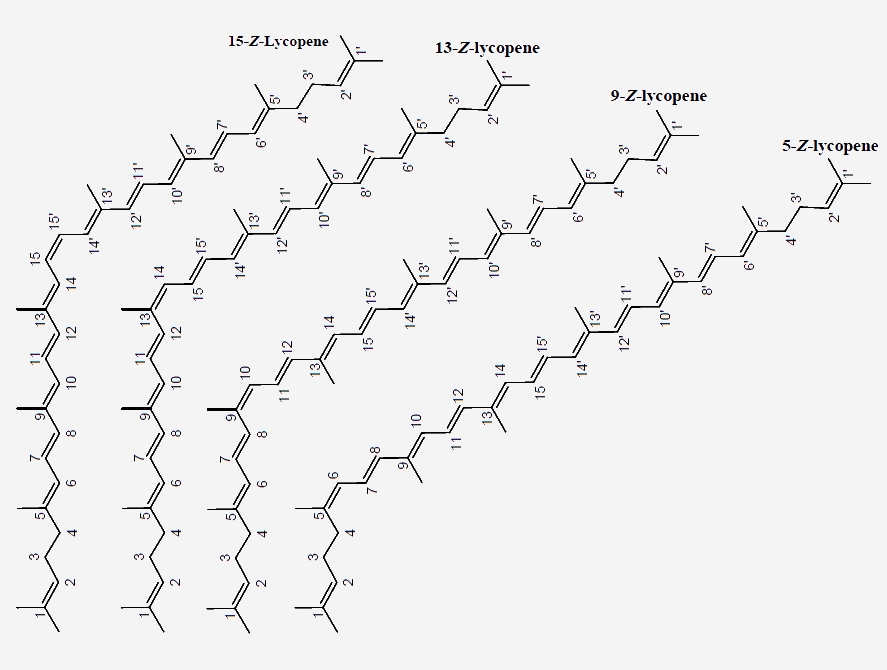
In principle, each carbon-carbon double bond in the polyene chain of carotenoid molecules may exist in two isomeric configurations of E (German: Entgegen, meaning “opposite”) and Z (Zusammen, meaning “together”). In various fresh fruits and vegetables, lycopene is predominantly found in the structurally stable E-from, while significant amounts of the Z-lycopene isomers are encountered in processed and stored products due to exposure to oxidative conditions. Depending on the degree of exposure to adverse conditions (e.g. oxygen, heat, light, metals, etc.), isomerization of seven out of the eleven carbon-carbon conjugated double bonds of lycopene gives rise to various mono-Z- or poly-Z-isomers.

The isomerization of lycopene causes significant alterations in its physical properties (e.g. light absorption, melting point, color intensity), chemical properties (e.g. antioxidant effects) and biological properties (e.g. bioaccessibility and bioavailability). Moreover, Z-isomers of lycopene have a higher absorption rate in the human body, which is probably mediated by their high polarity and improved solubilization in micelles, compared to their E-counterparts.
Lycopene is found abundantly in ripe red tomatoes, rosehips, watermelons, gac fruit, guavas and papayas. In these raw fruits and vegetables, 80-97% of the lycopene occurs in the all-E-form in the crystalized state. However, in most thermally processed foods, Z- isomers are found in significant amounts (I.e. 35% 15-Z-lycopene in thermally processed tomato products), due to the heat mediated isomerization. Significant 5-Z-, 9-Z-, 13-Z-, and 15-Z-lycopene contents have been reported in raw and processed tomatoes.
A series of multiple processing steps including hot/cold breaking, evaporation, pasteurization, sterilization and canning are involved in tomato processing to inactivate microorganisms, soften the tissue, and decrease the water content.
Owing to the highly unsaturated structure of lycopene and other carotenoids, they are susceptible to oxidation during the various food processing steps and during storage. In particular, the increased surface area of diced or dehydrated products enables greater exposure to oxygen, resulting in accelerated oxidation. Considering the sensitivity of lycopene, knowledge of the chemical reactions that contribute to oxidative degradation of lycopene and carotenoids is regarded as fundamental for developing strategies in order to avoid or at least minimize oxidation during handling, processing and storage of food.
 In hot break processing, temperatures in the range of 85-95°C for 1 to 5 min are usually used to inactivate pectinolytic enzymes (e.g. endopolygalacturonase and pectin methylesterase) and lipoxygenases that affect the structure of the plant and the aroma, respectively.
In hot break processing, temperatures in the range of 85-95°C for 1 to 5 min are usually used to inactivate pectinolytic enzymes (e.g. endopolygalacturonase and pectin methylesterase) and lipoxygenases that affect the structure of the plant and the aroma, respectively.  Lower temperatures in the range of 65-75°C are normally used in cold break processing to maintain the activity of these enzymes and to reduce the viscosity and increase the aroma of processed products, which are highly valued in certain products such as tomato juice.
Lower temperatures in the range of 65-75°C are normally used in cold break processing to maintain the activity of these enzymes and to reduce the viscosity and increase the aroma of processed products, which are highly valued in certain products such as tomato juice.In studies carried out by Koh et al., lycopene was stable during the initial thermal treatment (hot break 93°C for 5 min) but decreased by 20% through evaporation (63-79°C) and sterilization (100°C for 3-5 min) treatments during the preparation of tomato paste. From a nutritional perspective, cold break processing used during tomato paste and juice preparation is useful for the preservation of lycopene and other micro-constituents of tomatoes, since less heat damage will be imposed on these nutrients.
Tomato processing in general, and fruit-breaking and separation of skin and seeds from the pulp in particular, are operations that can significantly reduce the levels of some nutritionally important metabolites. For example, a gradual and significant decrease in lycopene content (ranged between 19 and 42%) was reported after processing of tomatoes into paste. In this study, 146.0 and 61.9 mg/100 g (dry weight) of all-E-lycopene was recorded in whole fruits, seeds and skin, respectively, which shows that the separation or removal of skin and seeds during processing eliminates significant amounts of carotenoids. Furthermore, in the same study, a modest decrease in lipophilic and hydrophilic antioxidant capacities was recorded during tomato processing from fresh fruit to paste, probably due to the significant losses of vitamin C (~50%), lycopene and other carotenoids. The best antioxidant agents in tomato were found to be in the fruit skin. Thus, the separation of skin and seeds from the pulp before processing leads to a significant loss of the antioxidant potential. To overcome this limitation posed by processing requirements, it is advised that the various antioxidant compounds available in tomato skin and seeds, including lycopene and phenolic compounds, be extracted from the processing waste and added back to the processed products in order to enhance its health benefits.
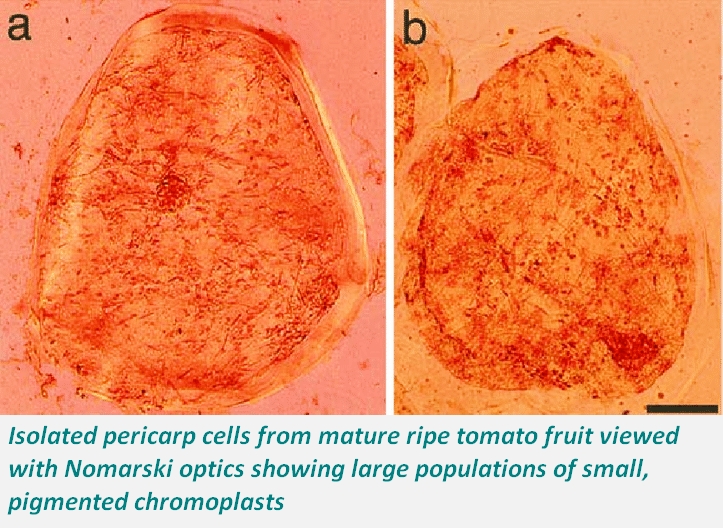
Researchers demonstrated that crystalline structures facilitate resistance to lycopene degradation and isomerization. Several thermodynamic and kinetic equilibria studies have showed that the processing conditions, food matrix, and physical forms of lycopene present in food determine the extent of lycopene isomerization and degradation. In tomatoes and several other fruit chromoplasts, lycopene aggregates into crystalline structures that provide a stable configuration and confer resistance to degradation and isomerization. Even in processed red tomato products, the physical form of lycopene (e.g., crystalline or non-crystalline) was found to influence the thermal stability significantly.
Reference: Chemical Stability of Lycopene in Processed Products: A Review of the Effects of Processing Methods and Modern Preservation Strategies. Ramesh Kumar Saini, Alaa El-Din A. Bekhit, Shahin Roohinejad, Kannan R.R. Rengasamy, and Young-Soo Keum. Journal of Agricultural and Food Chemistry 2020 68 (3), 712-726






































